Our body is made up of very complex proteins. In fact, it is not just us that is made up of very complex proteins. It is more accurate to say that all the living things are made up of very complex proteins. There are two kinds to proteins – functional protein and structural protein. What is the difference? Well, the functional protein helps with the chemical reactions in our cells – but the structural protein is like what you can see with your eyes and touch. You can touch your skin, finger nail, hair, skin… and organs inside your body!
Why am I talking about these things?
Because I am about to let you know that your cells contain all the information about these proteins. The information is used to make and control every single things which are inside and outside your cell. It is like an encyclopedia, written in the language that our body understands. Where can we find the information then? The famous DNA – inside the nucleus of a cell.
The DNA is a long double helix molecule. Helix is the shape of a twisted ladder.

May be you have seen this picture before. The DNA is made up of thousands and millions of a single unit called NUCLEOTIDE.
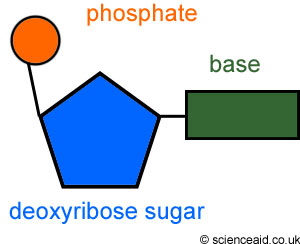
Nucleotides have another name – Nucleic acid. This is because we often have to see things from chemistry perspective to understand their property as a molecule.
DNA and RNA is all made up of nucleic acids. But only difference is that DNA is like a ‘REFERENCE COPY’ book in the library. As you know these REFERENCE COPY books cannot be taken out side the library. So, what do you do when you need an information from the book? You would photocopy the pages you need wouldn’t you? The RNA is like the photocopied pages which can take information from DNA in the nucleus to the cytoplasm to make proteins. This is because making protein needs big chemical reaction and this reaction can only take place in the cytoplasm.
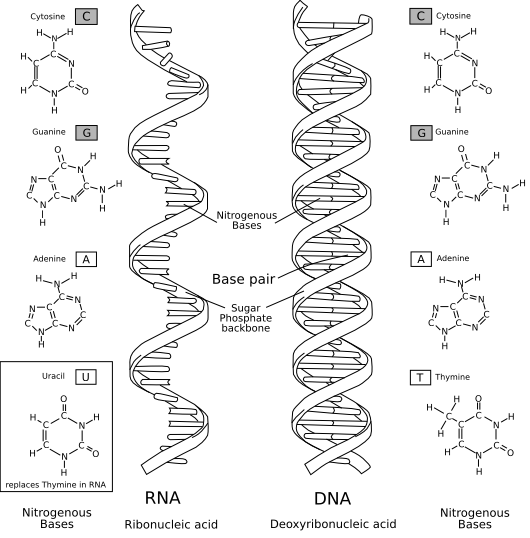
This is how RNA and DNA looks like. As you can see RNA is single stranded and DNA is doubles stranded. The part you cannot see is the difference in length. DNA is way way longer than RNA because it contains whole information, unlikely, RNA is way way shorter than DNA because RNA only contains copy of a section in a DNA strand.
You will actually more things about RNAs and DNAs when you take senior Biology subject. Hope this helps for you to kick start! 










